Are you ready to find 'reaction rate homework'? You can find all the information on this website.
Table of contents
- Reaction rate homework in 2021
- A study of reaction rates is called chemical
- Rate of reaction worksheet igcse
- Rates of chemical reactions
- Factors affecting rate of reaction worksheet pdf
- Rate of reaction ppt
- Reaction rates worksheet pdf
- Rates of reaction questions and answers pdf
Reaction rate homework in 2021
 This image shows reaction rate homework.
This image shows reaction rate homework.
A study of reaction rates is called chemical
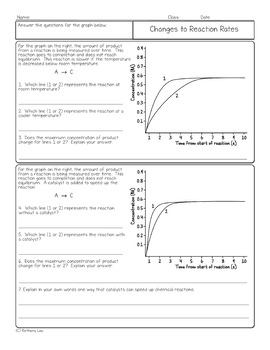 This picture representes A study of reaction rates is called chemical.
This picture representes A study of reaction rates is called chemical.
Rate of reaction worksheet igcse
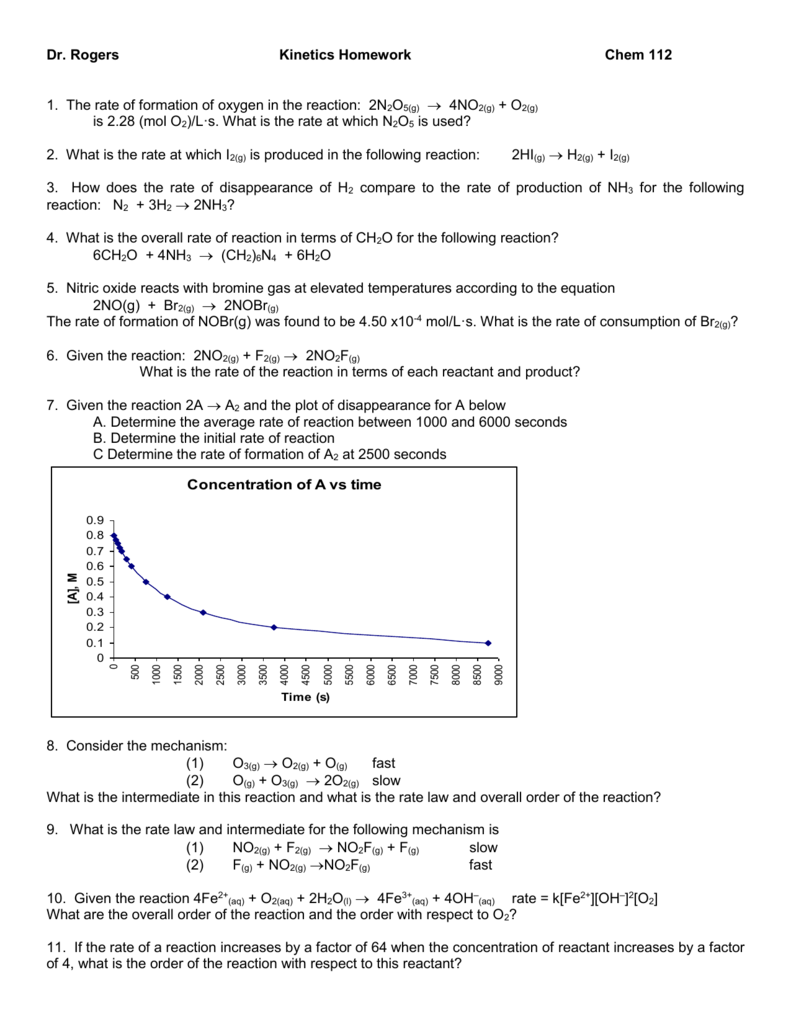 This picture demonstrates Rate of reaction worksheet igcse.
This picture demonstrates Rate of reaction worksheet igcse.
Rates of chemical reactions
 This image demonstrates Rates of chemical reactions.
This image demonstrates Rates of chemical reactions.
Factors affecting rate of reaction worksheet pdf
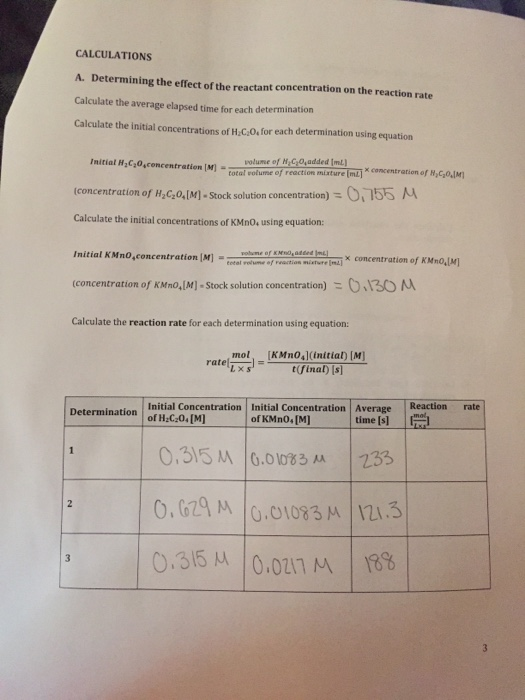 This picture representes Factors affecting rate of reaction worksheet pdf.
This picture representes Factors affecting rate of reaction worksheet pdf.
Rate of reaction ppt
 This picture demonstrates Rate of reaction ppt.
This picture demonstrates Rate of reaction ppt.
Reaction rates worksheet pdf
 This picture shows Reaction rates worksheet pdf.
This picture shows Reaction rates worksheet pdf.
Rates of reaction questions and answers pdf
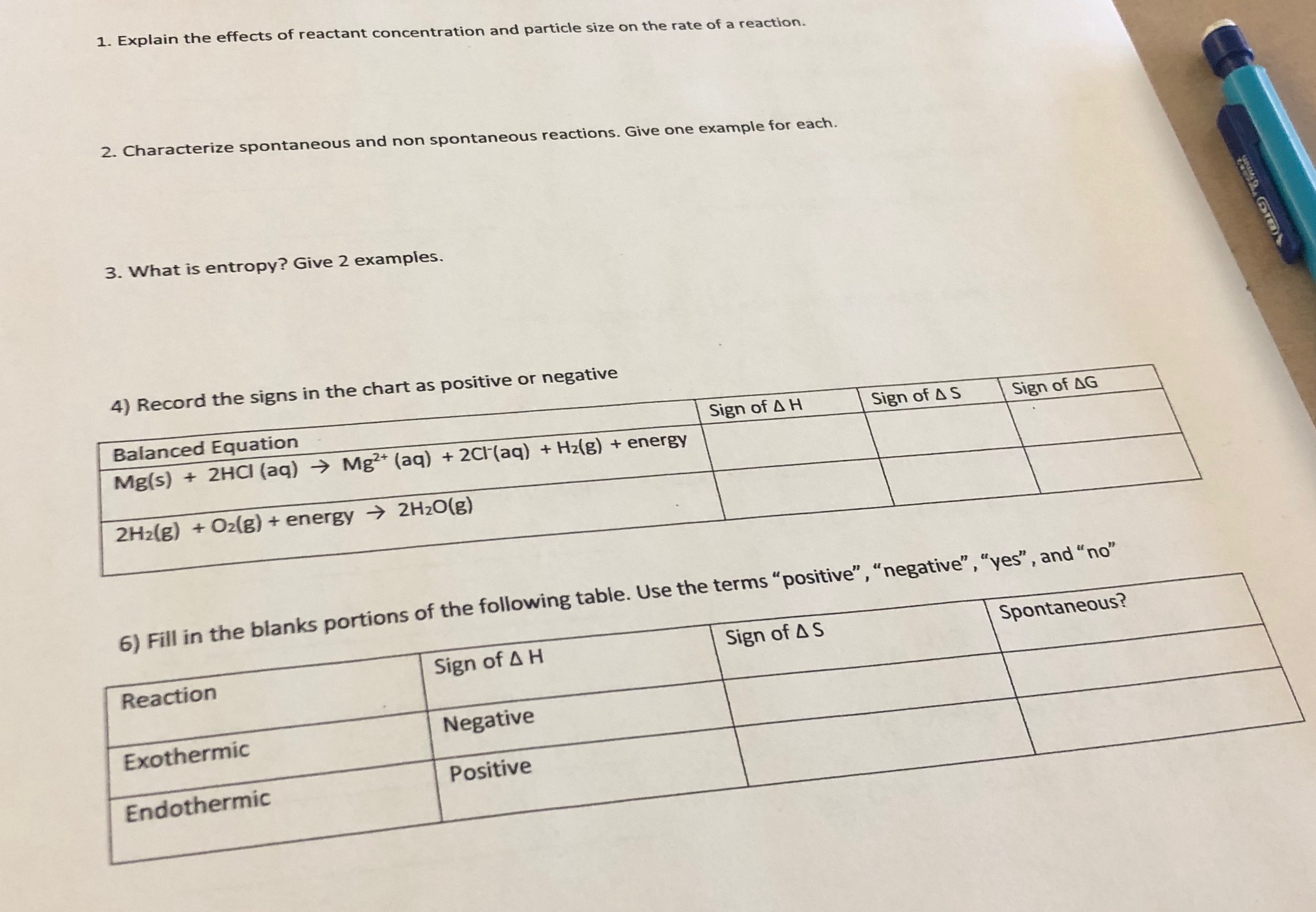 This picture shows Rates of reaction questions and answers pdf.
This picture shows Rates of reaction questions and answers pdf.
Which is the rate coefficient orrate constant of the reaction?
k is the rate coefficient orrate constant of the reaction. Stoichiometry is a branch of chemistry that deals with the relative quantities of reactants and products in chemical reactions. In a balanced chemical reaction, the relations among quantities of reactants and products typically form a ratio of positive integers.
How to calculate the rate of a reaction?
The rate law of a reaction, A arrow B + C is given by the expression: Rate = k, where k = 0.0113 mol/L min. If the initial concentration of the reactant is 0.225 mol/L, how long does it take for th... Consider the reaction A + 2B ---> P.
How to use rate equation and stoichiometry?
Rate Equation, Stoichiometry And Rate Laws Homework Help. In a balanced chemical reaction, the relations among quantities of reactants and products typically form a ratio of positive integers. Stoichiometry rests upon the very basic laws that help to understand it better, i.e., law of conservation of mass, the law of definite proportions (i.e.,...
How to calculate the enzyme reaction rate ( PPT )?
[1] Sample Calculation: Show your enzyme reaction rate calculation for Trial 1. [2] Convert enzyme concentrations of 2.5%, 5.0%, and 10% to ppt units. [3] For Procedure I/Table I, identify: [4] Draw a graph of the independent variable on the x-axis (abscissa) and the dependent variable on the y-axis (ordinate).
Last Update: Oct 2021